Alpelisib FDA Approval (2019)
May 2019What Happened
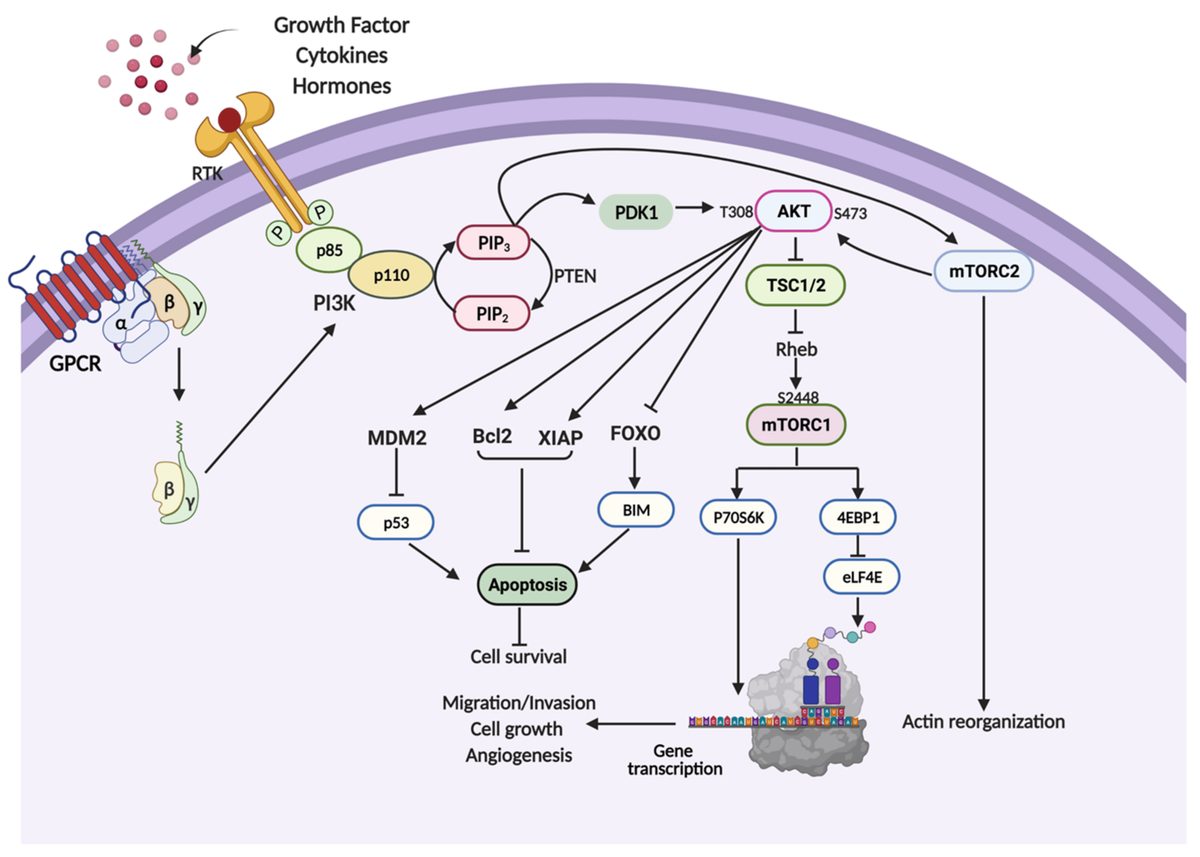
The FDA approved alpelisib (Piqray), the first PI3K inhibitor for breast cancer, based on the SOLAR-1 trial showing improved progression-free survival in PIK3CA-mutated patients. However, over 35% of patients experienced grade 3-4 hyperglycemia, and many required dose reductions or discontinuation.
Outcome
Alpelisib became standard of care for PIK3CA-mutated breast cancer but with significant safety management requirements.
The approval validated PI3K as a therapeutic target while highlighting that single-node inhibition triggers compensatory pathway activation and substantial toxicity. This set the stage for multi-node approaches like PIKTOR.
Why It's Relevant Today
PIKTOR's multi-node strategy specifically addresses alpelisib's limitations—targeting PI3K-alpha, mTORC1, and mTORC2 simultaneously to prevent pathway escape while using dietary intervention to manage metabolic side effects.