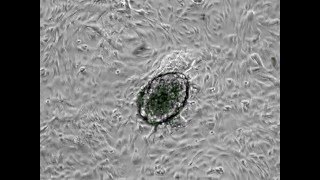
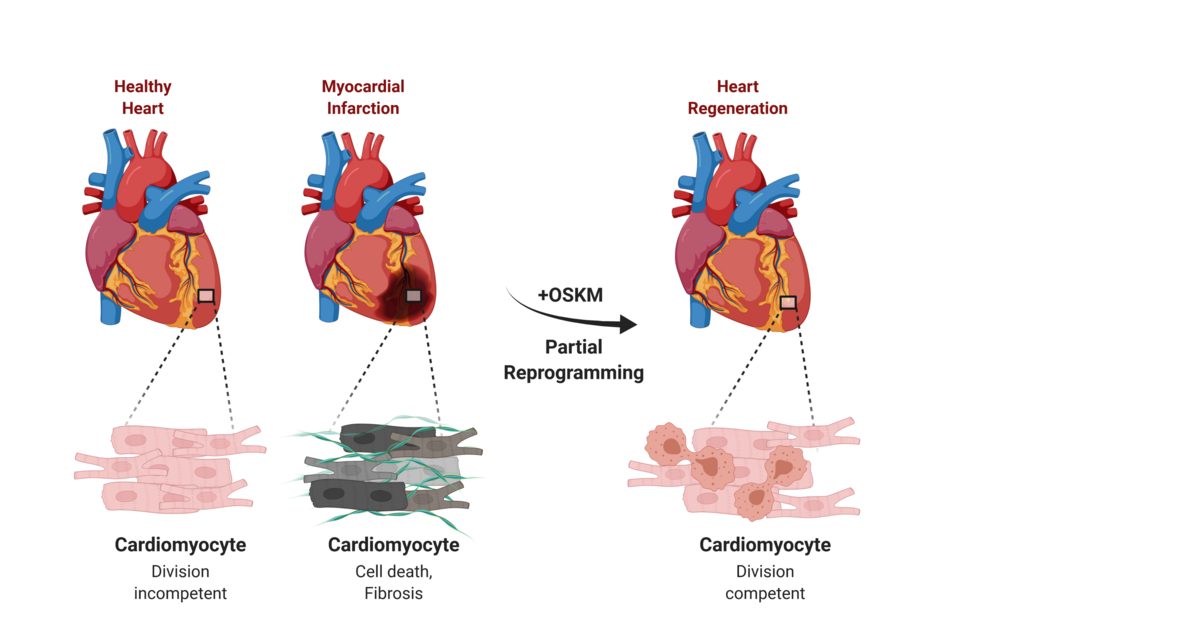
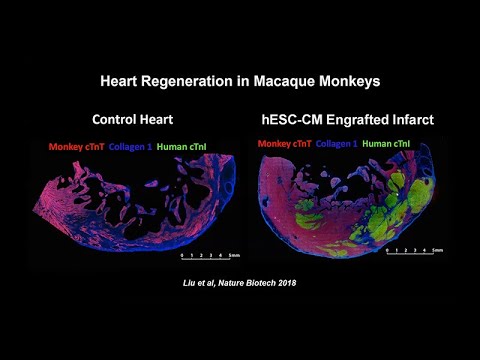
Researchers just demonstrated they can regenerate heart muscle using reprogrammed stem cells—and for the first time, proved these patches work in a human patient. In January 2025, a 46-year-old woman with advanced heart failure received 10 patches containing 400 million stem cell-derived heart muscle cells. Three months later, when she received a transplant, examination of her original heart revealed the patches had survived, formed blood vessels, and integrated with her heart tissue. When your heart suffers a heart attack, scar tissue normally replaces dead muscle cells permanently. Adult human hearts renew less than 1% of their cells per year. This damage has been irreversible—until now.
Researchers just demonstrated they can regenerate heart muscle using reprogrammed stem cells—and for the first time, proved these patches work in a human patient. In January 2025, a 46-year-old woman with advanced heart failure received 10 patches containing 400 million stem cell-derived heart muscle cells. Three months later, when she received a transplant, examination of her original heart revealed the patches had survived, formed blood vessels, and integrated with her heart tissue. When your heart suffers a heart attack, scar tissue normally replaces dead muscle cells permanently. Adult human hearts renew less than 1% of their cells per year. This damage has been irreversible—until now.
Scientists are closing in on therapies that could rewrite that script. They're discovering immune mechanisms that trigger regeneration in newborns, coaxing ordinary cells into beating heart muscle, and engineering patches of living tissue delivered through tiny chest incisions. A January 2026 breakthrough revealed CD4+ regulatory T cells control a key protein (MRG15) that enables neonatal hearts to regenerate—offering a potential vaccine-based approach to reactivate dormant repair pathways in adults. With 19.8 million people dying annually from cardiovascular disease and clinical trials now enrolling patients in Germany, this isn't just lab curiosity—it's a race approaching the finish line.