1
FDA Approves Anaphylm, Second Needle-Free Option Reaches Market
Discussed by: HCPLive, Allergic Living, and industry analysts covering the January 2026 PDUFA date
The FDA approves Anaphylm despite the identified deficiencies, determining they can be addressed through labeling or post-marketing commitments. Aquestive launches the sublingual film in 2026, giving patients two needle-free options: nasal spray and oral film. Competition between neffy and Anaphylm could drive down prices and accelerate adoption of needle-free delivery.
2
FDA Issues Complete Response Letter, Requests More Data
Discussed by: Fierce Biotech, Patient Care Online, and pharmaceutical industry analysts
The January 9 deficiency notification signals deeper issues than expected. The FDA issues a Complete Response Letter requiring additional clinical data, manufacturing changes, or labeling revisions. Approval slips to late 2026 or 2027, leaving neffy as the sole needle-free option and reducing competitive pressure on pricing.
3
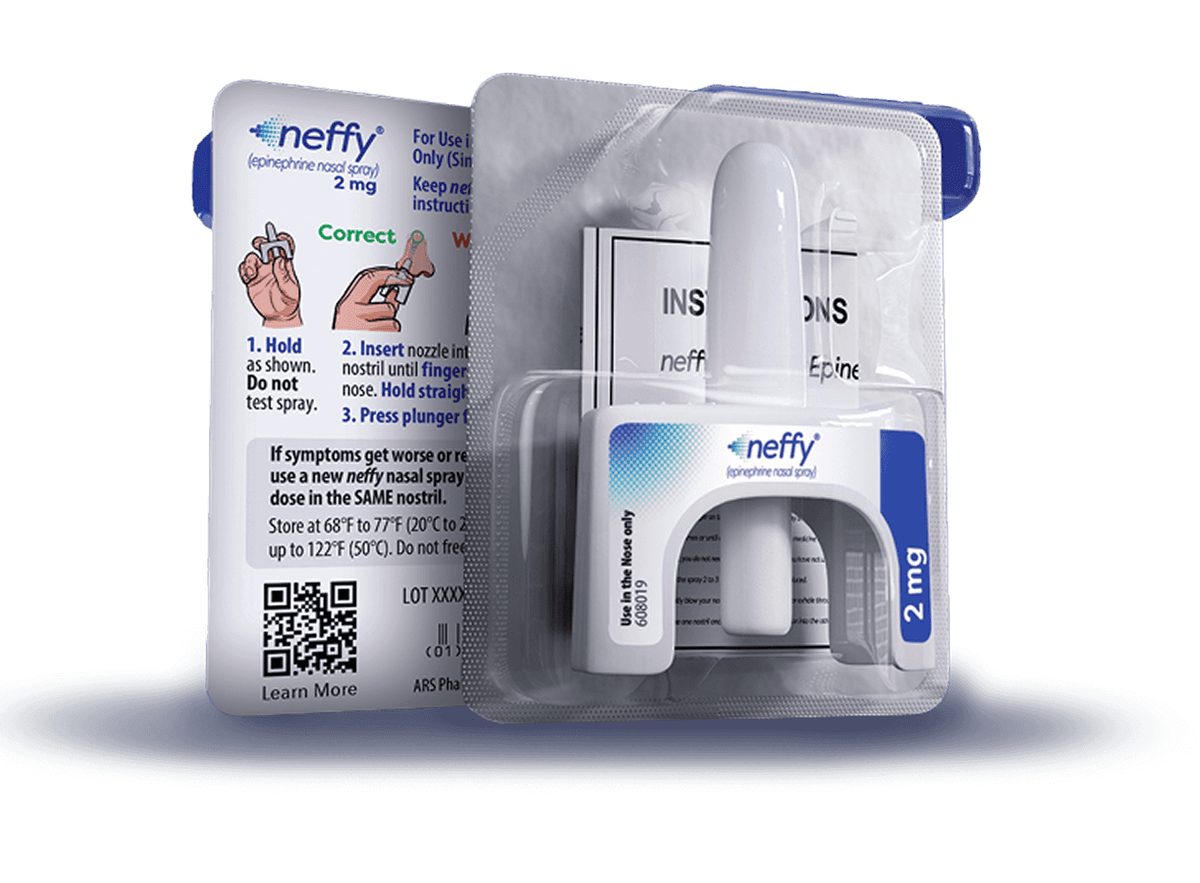
Anaphylm Approved, But Nasal Conditions Limit Neffy's Market Share
Discussed by: Yale Medicine, allergy specialists noting neffy's contraindications
Both products reach market, but their different delivery methods create distinct patient populations. Neffy's warnings about nasal polyps and nasal surgery push some patients toward Anaphylm's sublingual film. The oral film captures patients who cannot use nasal spray, while neffy retains those comfortable with nasal delivery.
4
Needle-Free Options Remain Niche as Insurance Coverage Lags
Discussed by: GoodRx, Consumer Reports, and patient advocacy groups
Even with FDA approval, high list prices and limited insurance coverage keep needle-free options out of reach for many patients. Generic EpiPen alternatives at $150-200 remain the default choice for cost-conscious consumers, and needle-free adoption remains concentrated among patients with documented needle phobia or ability to pay out of pocket.
5
Delayed Approval Extends Neffy's Market Exclusivity
Discussed by: Industry analysts, Seeking Alpha contributors following AQST stock
The FDA's January 9 deficiency notification delays Anaphylm approval into late 2026 or 2027, giving neffy 12-18 additional months as the sole needle-free option. ARS Pharmaceuticals uses this time to establish market dominance, secure preferred formulary positions with insurers, and build brand recognition. By the time Anaphylm launches, neffy has already captured significant market share and established physician prescribing patterns that prove difficult to disrupt.
6
Anaphylm Resubmission Approved in Q4 2026, Two Needle-Free Options Compete
Discussed by: Aquestive management, pharmaceutical analysts tracking AQST stock
Aquestive successfully completes human factors and pharmacokinetics studies by Q3 2026 and resubmits the NDA. The FDA grants approval in Q4 2026, bringing Anaphylm to market alongside neffy. Both products establish distinct market positions—neffy for nasal delivery, Anaphylm for sublingual administration—reducing reliance on needle-based epinephrine and creating competitive pricing pressure.
7
European Approval Precedes U.S. Launch, Anaphylm Gains International Foothold
Discussed by: Aquestive investor relations, European regulatory analysts
Anaphylm receives European marketing authorization in late 2026 before U.S. approval, allowing Aquestive to establish commercial presence and clinical experience in Europe. This international success strengthens the company's position for eventual U.S. launch and demonstrates product viability to U.S. physicians and payers.